
Research and Development
Research initiatives at Carilion Clinic are patient-centered. Here, participating patients, their care teams and our researchers work together to develop new medicines, innovative devices and advanced procedures. Explore more than 135 clinical trials in more than a dozen different clinical spe...
About Us
Our Mission
Research is not just an important part of the work we do at Carilion Clinic. Our commitment to research is, along with patient care and education, one of the three essential pillars supporting our mission to improve the health of the communities we serve.
Research initiatives at Carilion are patient-centered. Here, participating patients, their care teams and our researchers work together to develop new medicines, innovative devices and advanced procedures that may benefit them—and countless others—in the years to come.
With more than 100 clinical trials in process at Carilion at any given time, patients in our region have many opportunities to participate in this vital work.
We could not reach our mission without clinical research—or without the participation of our patients.
Our Focus
Together with the Institutional Review Board, Health Analytics Research Team and Research Compliance, Research & Development department (R&D) is responsible for oversight of all research projects at Carilion Clinic. R&D manages administrative aspects such as contracting, finance, regulatory support, grant management and research coordination.
To meet our goal of increasing both the quality and the quantity of our research studies, we focus equally on trial participants, ongoing education, streamlining processes and increasing collaboration.
That collaborative spirit, which puts patient participants at the center of our research, also guides our dedication to innovation within Carilion Clinic, a steadfast investment in the health of the surrounding community and a growing list of prestigious national and statewide partnerships.
In particular, R&D collaborates on many projects with the Virginia Tech Carilion School of Medicine and the Fralin Biomedical Research Institute at VTC, and our association with the integrated Translational Health Research Institute of Virginia (iThriv) expands our research footprint throughout the commonwealth.
Our research team continually looks for new ways to strengthen existing partnerships and foster new opportunities to further Carilion’s mission—and to move medicine forward.
Rewards of Research
Our Rewards of Research video series features examples of how clinical trials connect the community with research opportunities.
Research and Development News
News from Research and Development, the Office of Human Subjects Research Protections, and the Health Analytics Research Team.
February 2026 Newsletter
December 2025 newsletter
November 2025 newsletter
October 2025 newsletter
July 2025 newsletter
May 2025 newsletter
January 2025 newsletter
December 2024 newsletter
October 2024 newsletter
August 2024 newsletter
April 2024 newsletter
February 2024 newsletter
October 2023 newsletter
August 2023 newsletter
June 2023 newsletter
April 2023 newsletter
Feb. 2023 newsletter
Jan. 2023 newsletter
Nov. 2022 newsletter
Sept. 2022 newsletter
Aug. 2022 newsletter
July 2022 newsletter
May 2022 newsletter
April 2022 newsletter
March 2022 newsletter
Feb. 2022 newsletter
Clinical Trials
Carilion Clinic conducts clinical trials in three main areas—investigational drugs, biologics and devices—across 18 clinical areas ranging from cancer and cardiovascular studies to pediatrics and surgical techniques.
Our Research Team
Meet our directors and our team of research administrators, coordinators and assistants.

Services and Resources
Services Provided
Click below to learn more about the services we provide.
Research and Development Application
To submit a Research and Development application, click below.
Research Disclosure Information
In accordance with regulatory requirements, this web page provides public access to information pertaining to conflicts of interest (COIs) of externally funded or supported research.
The information on this web page will remain available for three (3) years after its most recent update. Information provided will include:
- The Covered Individual’s name
- The Covered Individual’s title and role with respect to the research
- The name of the entity in which the financial interest is held
- The nature of the financial interest that constitutes a financial COI
- The approximate value of the financial interest by range or, if the dollar value cannot be determined by reference to public prices or other reasonable measures of fair market value, a statement to that effect
A management plan is established in cooperation with the Covered Individual to address the COI per Carilion Clinic policy. The information posted on this website is derived from the management plan.
The information posted on this website is subject to updates on at least an annual basis and within 60 days of Carilion Clinic’s identification of the COI.
For more information or questions regarding any conflict of interest disclosures, please contact the Organizational Integrity and Compliance office at compliance@carilionclinic.org or 540-510-4573.
Educational Resources
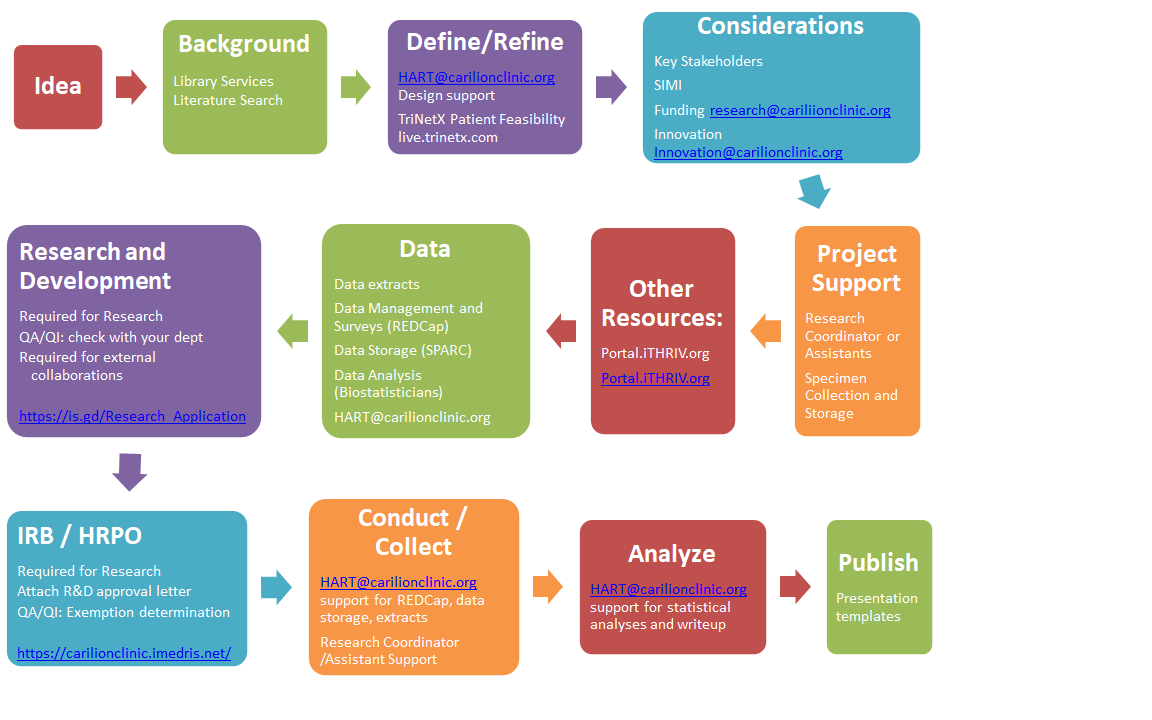
Need help navigating research or QA/QI processes at Carilion?
This new tool will provide as much or as little information and support as you want, based on questions you answer. In addition, you can track your progress and associated costs, and receive a PDF summary.
This figure presents an overview of the process, and we invite you to visit MyProjectPath for more in-depth information tailored to your specific project. Please contact HART@carilionclinic.org with questions.
Riverside 4 Research Suite

The Riverside 4 CRS serves as a multifunctional hub supporting a range of clinical research activities. Research personnel now have access to dedicated research space in the Riverside complex—study visit rooms, a specimen processing lab and hoteling space—allowing for efficiencies in clinical trial coordination and participants' experience.
Research Lab
Basic Science Research Lab
The lab fosters an environment where medical questions can lead to answers that impact the care we provide our patients.

Research Acceleration Program
Carilion Clinic’s Research Acceleration Program (RAP) funds Carilion employees interested in pursuing clinical, biomedical, or behavioral research projects. Projects of interest are those that would advance or contribute to Carilion’s care objectives, education, and research excellence, as well as strengthen Carilion’s collaborative relationships across disciplines within Carilion and/or with research institutions within the region.
While all significant, innovative, and rigorous research proposals are welcomed, applicants are encouraged to pursue research that aligns with our Vision 2025 Research and Innovation goals:
- Expand, design, and invest in care delivery of the future.
- Grow and invest in clinical platforms that provide cutting-edge treatment capabilities
- Deepen academic partnerships with:
• Virginia Tech
• Fralin Biomedical Research Institute at VTC
• Radford University Carilion
Instructions can be found in the RAP25 guidelines below.
Important Dates and Information
Tier I Process
- Tier I applications are due Monday, December 2, 2024, at 5 p.m. EST.
- Applicants selected for funding will be notified by January 22, 2025.
- Awards will be up to $40,000.
Tier II and III Processes
- Final applications are due Monday, December 2, 2024, at 5 p.m. EST.
- Applicants selected for funding will be notified by January 22, 2025.
- Awards will be up to $15,000 (Tier II) and $2,500 (Tier III).
For questions and inquiries, contact Melissa Mercure at mzmercure@carilionclinic.org; Vera Hollen at vlhollen@carilionclinic.org; Francis Farrell at fxfarrell@carilionclinic.org or research@carilionclinic.org.
Research Day 2025
April 14th, 2025
7:00-10:00 oral presentations (virtual via Teams)
11:00-1:00 pm Research/ Fellows Poster Session
2:00-4:00 pm Faculty/ Staff and Student Poster Session
The registration link for the 2025 Carilion Clinic Research Day is now open. Research Day is a forum for our researchers and their external collaborators to highlight the work conducted in our hospitals, clinics, basic science laboratories, and in the community. Researchers will have the choice to present their work as a poster or via oral presentation via Teams. In the past, over 100 abstracts comprised of research, case studies, and quality projects were submitted.
Abstracts are due by March 28th at 5:00 p.m. Poster and/ or oral presentation acceptances will be communicated by March 31st, 2025. This year, the poster session will be held at Fralin Biomedical Research Institute, Riverside 4 G-102 A and B. Residents and Fellows will present posters from 11:00- 1:00, followed by Students and Faculty/ Staff from 2:00-4:00. Oral Presentations will be scheduled between 7:00 a.m. and 10:00 a.m.
If you have any questions, contact Research@carilionclinic.org or Francis Farrell, fxfarrell@carilionclinic.org.
Community Engagement Studies
In addition to clinical trials to investigate new therapies and treatments, Carilion Clinic also conducts community-engaged studies. These studies are also known as Team Science because the health care provider is engaged with the community or organizations outside of the walls of a hospital. In most cases, these studies explore problems that affect the community at large such as high blood pressure. A recent seroprevalence study at Carilion Clinic attempted to determine the number of individuals who were exposed to COVID-19 even if they did not exhibit the symptoms of COVID-19 infection.

Research Partners

All research programs have at least one Carilion Clinic employee as the principal investigator. Collaboration is critical to providing the most advanced opportunities for scientific discoveries.
Fast Facts
Advancing Research Through Growth and Expansion
At Carilion Clinic, we base everything we do on three pillars: patient care, education and research. Together, our commitment to these equally vital areas helps us reach our mission to improve the health of the communities we serve.
